TCD Researchers Contribute to Understanding Human Transmembrane Signalling
Posted on: 12 October 2011
New international collaborative research led by Stanford University, the University of Michigan and involving Trinity College Dublin recently published in Nature, increases our understanding of how a human transmembrane protein known as β2-adrenergic receptor, a G protein-coupled receptor (GPCR), works at a molecular level.
There are over 750 GPCRs distributed throughout our tissues with representatives in almost every cell type. About a third of drugs on the market today target GPCRs. The GPCRS play key roles in heart and lung function, in how we respond to hormones and neurotransmitters and by extension they influence mood and behaviour, and are involved in immunity and inflammation.
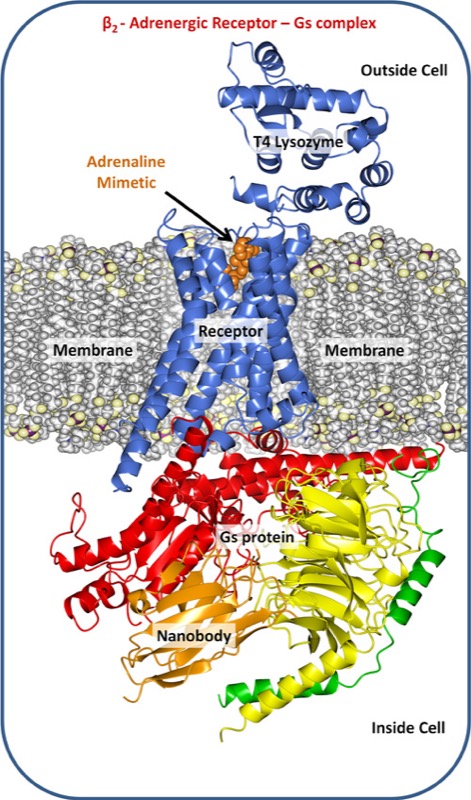
The fight-or-flight hormone adrenaline mediates its activity by way of a particular GPRCR, the β2 adrenergic receptor. When the receptor grabs hold of adrenaline shot into the blood by the adrenal gland it is immediately activated. The formation of this receptor-G protein complex triggers a series of reactions within and between cells that are part of the body’s response to adrenaline.
Trinity’s Membrane Structural and Functional Biology Group affiliated with the Schools of Medicine and Biochemistry & Immunology were involved in solving the crystal structure of the β2-adrenergic receptor-G protein complex. Crystal production involves the use of robots that dispense nano-litre volumes of a highly viscous, protein-laden lipidic liquid crystal or mesophase into home-built, multi-well glass sandwich plates for crystallisation screening. The mesophase mimics the lipid bilayer membrane in which the receptor resides in the cell. The particular lipid used in this study, synthesised and purified in the Trinity lab, was designed in the Group to create an environment that comfortably housed the receptor complex enabling it to assemble into an ordered array and to form a crystal.
The crystals typically are very small, just a fraction of a millimetre in size and are extremely fragile. They are harvested carefully from the mesophase, cryo-cooled in liquid nitrogen and then shipped in special Dewars to a synchrotron X-ray facility, the Advanced Photon Source, at the Argonne National Laboratories on the outskirts of Chicago. The synchrotron is a high-energy, particle accelerating machine about a mile in circumference that produces an extraordinarily intense beam of X-rays matched in size to that of the crystal. There, state-of-the-art technologies are used to centre the crystal in the laser-like X-ray beam and to collect diffraction data which, upon processing and modelling generated the structure published in Nature.
Trinity’s Professor of Membrane Structural and Functional Biology, Martin Caffrey in the Schools of Medicine and Biochemistry & Immunology explained: “New and improved drugs are always in demand. But to design them in a rational way we need to know how the receptor, in this case the β2-adrenergic receptor, is put together in a structural sense and how this structure enables it to function as a receptor and a communicator of information. By structure we refer to the arrangement in three-dimensions of the receptor’s constituent atoms and amino acids.”
“We would also like to know how this structure changes when the receptor binds adrenaline and how this facilitates downstream signalling. The only way to get such detailed information for a complicated membrane protein like the β2-adrenergic receptor is to use what is called macromolecular crystallography. This is a method that requires a well ordered crystal of the receptor. The crystals must then be irradiated with and scatter X-ray photons in a way that can be used to decipher the receptor’s structure.”
“One of the big challenges in this protracted and involved process or pipeline is to coax the protein into the regular and ordered lattice of a crystal. This is particularly difficult in the case of GPCRs because they continually flit about structurally in the plane of the membrane and, at any one moment, can be seen to exist in a number of different conformations or shapes. The particular conformation assumed, in turn, dictates the receptor’s biological activity. To get a collection of receptor molecules to crystallize, ideally they should all assume the one stance or conformation.”
“Professors Brian Kobilka and Roger Sunahara, who led this research project, devised clever strategies for locking the receptor-G protein complex into what amounts to a single conformation. One of these made use of an unusually small antibody, called a Nanobody, raised against the complex in llamas. So stabilised and rendered uniform, the receptor complex was successfully crystallised and its structure solved.”
This particular study, in concert with other work reported in this issue of Nature, provides the first high-resolution structural insights as to how these ubiquitous receptors work at a molecular level to sense a signal on the outside of a cell and to communicate it across the membrane to its cognate G protein which sets in motion myriad responses within the cell. The β2-adrenergic receptor complex is a paradigm for the larger family of GPCRs.
The project was led by Brian Kobilka, Professor of Molecular and Cellular Physiology at Stanford University School of Medicine and Roger Sunahara, Professor of Pharmacology, University of Michigan Medical School. Professors Kobilka and Sunahara assembled an interdisciplinary team from across the globe that included molecular biologists, protein biochemists and biophysicists, crystal growers and macromolecular crystallographers, and synthetic organic chemists. In all, 20 individuals from six institutions in the USA and Europe contributed to the work. Each played a complementary role in providing insights into the molecular workings of these medically and pharmaceutically important membrane protein receptor complexes. The TCD group contributing to the work include Tasadaque Shah, Joseph Lyons and Martin Caffrey. All are members of the Membrane Structural and Functional Biology group affiliated with the Schools of Medicine and Biochemistry & Immunology. The synthesis was done in Professor David Grayson’s laboratory in the School of Chemistry at TCD.
The MS&FB group is funded through Science Foundation Ireland, the USA National Institutes of Health and the European Union Framework Seven Programme.
